CALLING ALL INVESTIGATORS INTERESTED IN CONDUCTING COVID-RELATED RESEARCH!

The Alliance’s Biomedical Informatics, Bioinformatics, and Cyberinfrastructure Enhancement Core (BiBEC) is part of the national IDeA-CTR network, whose activities include involvement with the National COVID Cohort Collaborative (N3C) Data Enclave. The Alliance has an executed Data Use Agreement with the National Institutes of Health, meaning that researchers wishing to conduct research in COVID-related research may request access to this data resource. BiBEC is also involved with N3C research and education. The N3C Data Enclave was officially launched in September 2020 and is funded by the National Center for Advancing Translational Sciences (NCATS).
The National COVID Cohort Collaborative (N3C) data platform contains data on at least 1.6 COVID+ cases, representing almost 6 million persons. In 2023, the Alliance will begin to contribute Puerto Rico-specific COVID data to the N3C Enclave. COVID-19 clinical data is available for research through the new N3C analytics platform.
The N3C aims to aggregate, harmonize, and make accessible vast amounts of data from healthcare providers nationwide to accelerate advances in COVID-19 research and clinical care. With the uncertainty of the COVID-19 global pandemic, the scientific community and Clinical and Translational Science Awards (CTSA) Program created the N3C partnership to overcome technical, regulatory, policy, and governance barriers to harmonizing and sharing individual-level clinical data.
In addition to the vast amounts of data available for coronavirus research, the N3C also aims to help scientists analyze these data to understand the disease and develop treatments. The N3C enclave currently holds data related to roughly:
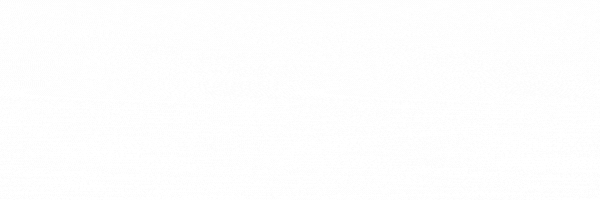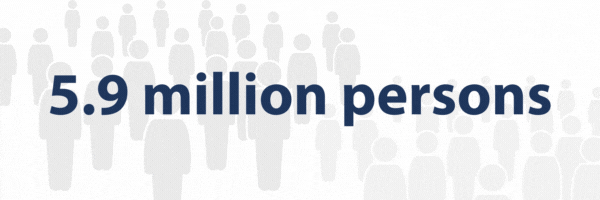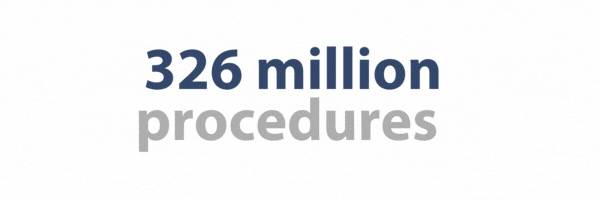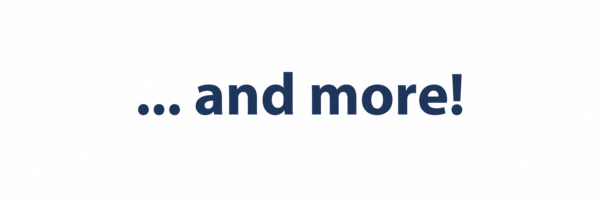
- 56 sites
- 5.9 million persons
- 1.6 million COVID+ cases
- 326 million procedures
- 582.8M million clinical observations
- 3.1 billion lab results
- …and more!
Benefits of Using the N3C Data Enclave!
- Generating sample data for pilot projects and grant proposals
- Training algorithms on a very large cohort of patients
- Opportunities to train junior researchers (the domain teams can offer support and a network for these investigators)
- Informatics and statistics opportunities for innovation and evaluation
- Opportunities for clinicians to investigate COVID-19 treatment patterns and disease progression
Three levels of data are available:
- Synthetic data or a safe data derivative,
- De-identified data; and
- Limited data.
If you request limited data, you will need IRB approval from the UPRMSC and must provide documentation of human subject research protection training.
The Alliance has an executed Data Use Agreement with NIH for access to the N3C Data Enclave. Interested investigators may REGISTER FOR ACCESS using their institutional email address. After you register for an account, access is granted within 48 business hours.
One-on-one support for registering for access is available through Dr. Mary Helen Mays, Leader for BiBEC and N3C Navigator for the UPR/Alliance (mary.mays@upr.edu).
Data Use Requests
A Data Use Request (DUR) is an online application submitted by the researcher within the N3C Data Enclave that describes the scope and nature of the project and justifies the requested data access tier. Approval of the DUR by the Data Access Committee (DAC) is required before a researcher can access data within the enclave. (Approved DURs are valid for 1 year.) Please contact the Alliance N3C Navigator, Dr. Mary Helen Mays (mary.mays@upr.edu) for assistance.
Domain teams are multidisciplinary research teams focusing on specific aspects of COVID-19 ranging from diabetes to pediatrics. These teams collaboratively submit data access requests to avoid duplication of efforts and to ensure appropriate expertise while answering questions about the pandemic. Joining a Clinical Domain Team also gives Investigators the opportunity to connect with other researchers currently working within the N3C Data Enclave in common areas of interest, opening up opportunities for collaboration. See the list of clinical domain teams.
Learn More about the N3C Initiative and the N3C Data Enclave at these Links
- Read a complete overview of the N3C enclave.
- Review the webinar slides or watch a recording of the October 28, 2020 presentation, “Building a COVID-19 Analytics Platform to Turn Clinical Data into Knowledge: Introducing N3C”
- More information on the N3C Data Enclave and general information about N3C are found in these tutorials. Additional can be found at these links: webinars and information sessions.
- Read the JAMIA paper: “The National COVID Cohort Collaborative (N3C): Rationale, Design, Infrastructure, and Deployment”.






